
Click Sign in through your institution.Shibboleth / Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.Ĭhoose this option to get remote access when outside your institution. Typically, access is provided across an institutional network to a range of IP addresses. If you are a member of an institution with an active account, you may be able to access content in one of the following ways: They are in fact very light, and carry the same amount of charge as the hydrogen ion, but exactly opposite because they are negative.Get help with access Institutional accessĪccess to content on Oxford Academic is often provided through institutional subscriptions and purchases. He found that mass to charge ratio was over a thousand times lower than that of a hydrogen ion (H+), suggesting either that the particles were very light or very highly charged. To do this he measured how far the ray was deflected by a magnetic field. In his 3rd experiment he wanted to see if he could measure the mass to charge ratio (mass divided by amount of charge). Light is not bent by electrical or magnetic fields. This is important as it shows that the rays are not the same as a beam of light. He found the rays did indeed bend, and in the direction expected for a negative charge. In his second experiment he wanted to see if the rays would bend in the presence of an electrical field, which is what you would expect for a charged particle.
He found that the rays were bent and the negative charge was bent exactly the same. Thomson set up his cathode ray tube with, but placed a magnet above the path of the rays. He knew that electrically charged objects can be deflected by magnets (Michael Faraday discovered this and is his theory of electromagnetism). In Thomson's 1st experiment he wanted to see if he could separate the negative charge out of the rays. They knew that some sort of ray was coming from the cathode (filament) and that there was some sort of negative charge emitted from the cathode too because an electrical current flowed in the circuit between the screen and the cathode.

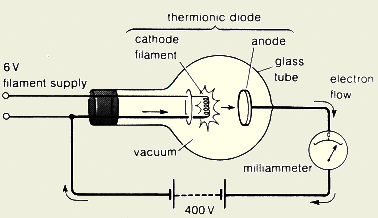
In reality the power needs to be very high though so you would use mains electricity converted to D.C.Īt the time Thomson started his work, the glow observed on the screen was mysterious and nobody knew what it was. You then connect a second battery with the (+) terminal connected to the screen and the (-) terminal connected to the filament. (It's hard to explain how it is wired up without drawing a picture! Think of it as the filament being connected to a battery - it will glow just like a light bulb but not as brightly. This puts electrical field between the screen and the filament - and if the screen is positive then electrons from the filament will stream towards the screen causing it to glow. At the same time you connect the filament and the fluorescent screen together with an electrical source. You pass an electrical current through the filament and it starts to glow.
#Cathode ray experiment summary tv#
At the other end is fluorescent screen which is just like an old fashioned TV screen. Inside at one end is an electrical filament (which is actually called the cathode in this experiment) just like the one inside a light bulb.

His experiments were all conducted with what is known as a cathode ray tube, so firstly I will try to explain what this is and how it works.Ī cathode ray tube is a hollow sealed glass tube which is under vacuum (has had all the air sucked out of it).


 0 kommentar(er)
0 kommentar(er)
